PYROPROCESS
A Carbon-Free Recovery Route for Pure Ti:
Metallic titanium is commonly produced by the carbon-dioxide-emitting Kroll process. The modern shift to sustainable technology has generated global interest in green processes.
Our laboratory focuses on the sustainability of the Ti production electrorefining process in conjunction with the regeneration process of reducing metal using an inert anode.
Energy consumption and CO2 emissions were 1.66 times less than those of the Kroll process.
Molten salt based electrorefining process
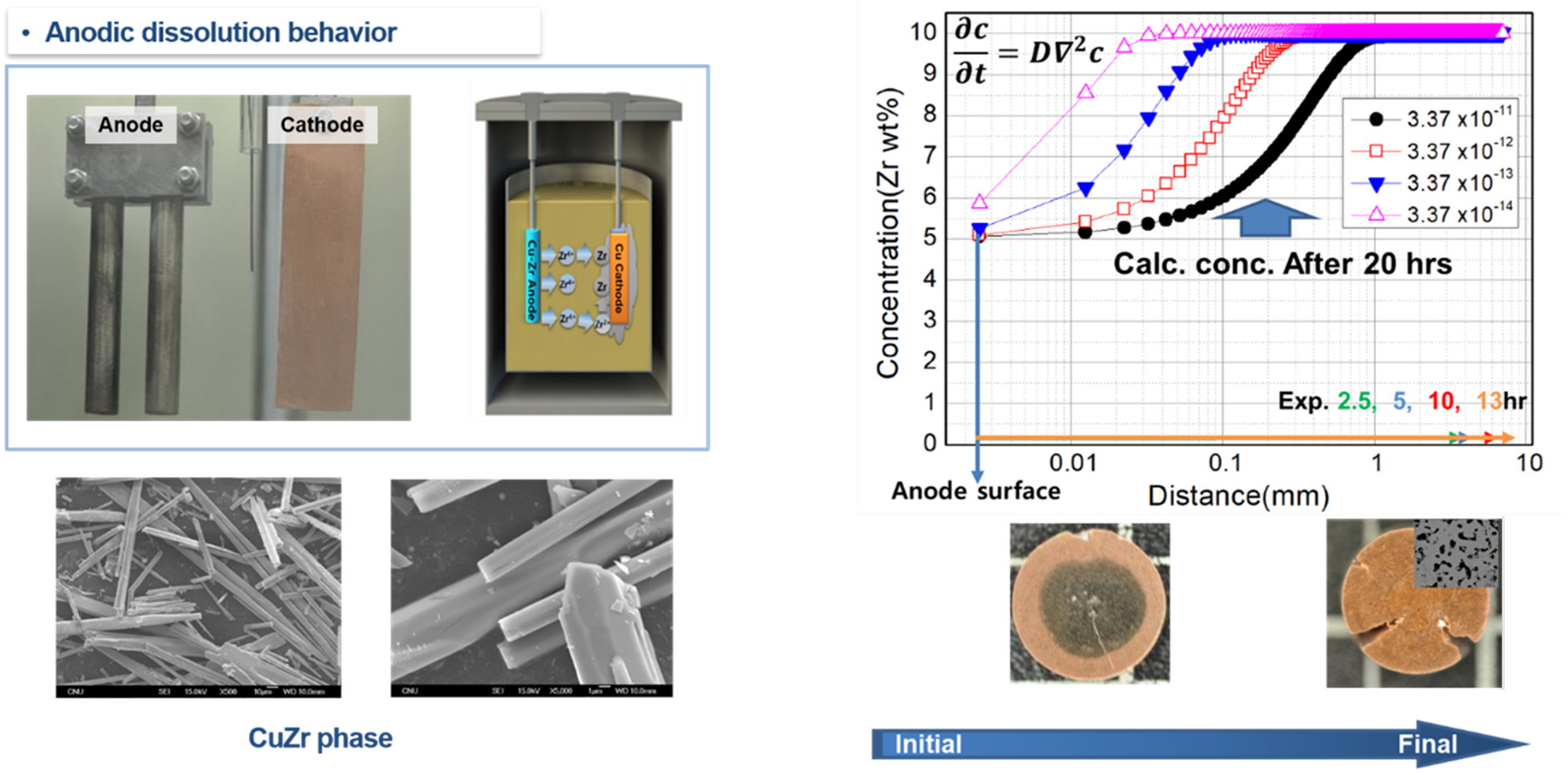
In order to understand the anode dissolution behavior from the solid anode, the dissolution rate was calculated under the assumption that anode dissolution occurs due to diffusion into zirconium, and compared with the experimental results.
Zr has a variable diffusion rate in Cu depending on the intermetallic phase, and in the case of the largest diffusion coefficient, the dissolution distance for 20 hours is only 1 mm from the surface. However, in the actual anodic dissolution process, 3mm is dissolved for 2.5 hours, and the dissolution depth for 13 hours reaches 8mm. In other words, it exhibits a diffusion behavior tens of times faster than the theoretical dissolution distance.
The SEM picture on the left is the data obtained by dissolving CuZr with acid and collecting only the CuZr phase. The Zr forms a columnar phase when solidified as CuZr alloy, and this microstructure forms a continuous channel when the anode is dissolved, and because Zr ion transfer from the inside through this channel, electrorefining can be performed without increasing the anode resistance.
Hot corrosion inhibition
The high-temperature molten salt dissolves the passivation film of most commercial materials and lowers the corrosion resistance. This is one of the most important factors that hinder the commercialization of the molten salt process.
In our laboratory, we study the corrosion behavior of metallic and ceramic materials in a high-temperature molten salt atmosphere, and conduct studies on new alloy development, surface treatment, and process conditions establishment to increase corrosion resistance.
Corrosion mechanism of Ni base superalloy in molten salt
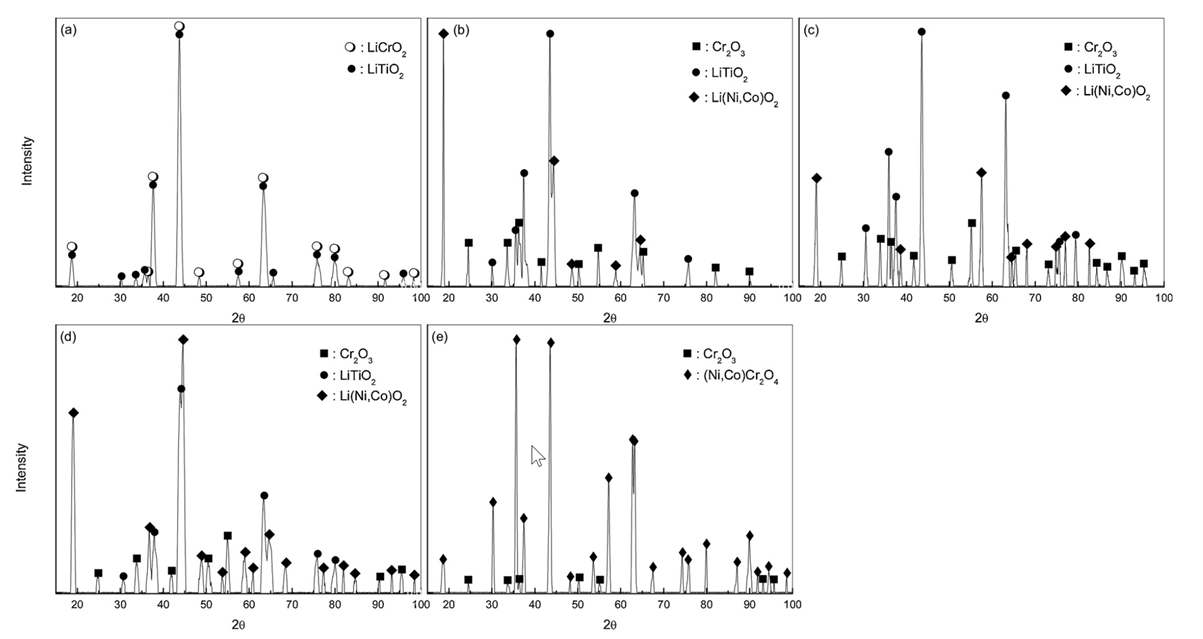
The effective life of the scale was confirmed by spallation and Cr depletion, leading to accelerated internal corrosion, and a dense and continuous Cr-rich oxide-scale layer was found to retard the inward diffusion of oxygen and the outward diffusion of the constituent elements via the scale layer.
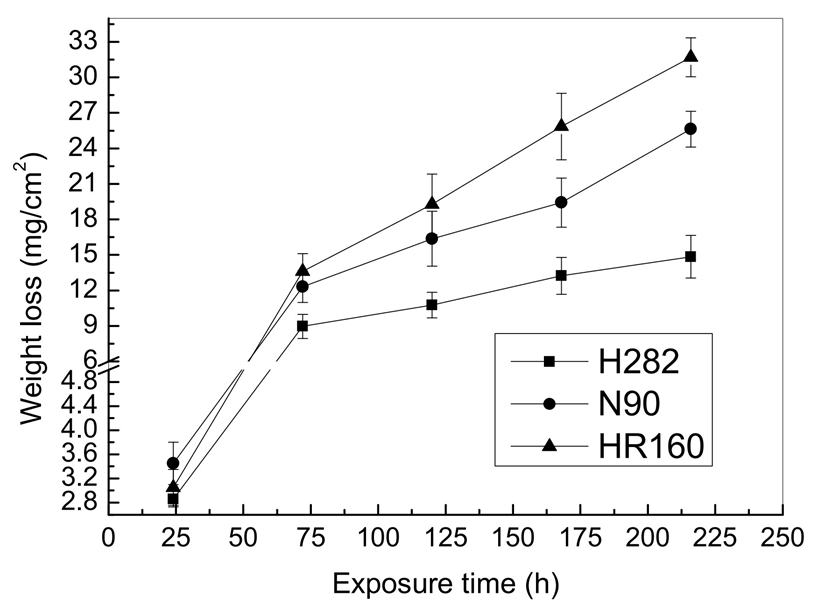
The hot corrosion behaviours of Ni-Co-based alloys in an oxidative LiCl-Li2O molten salt were investigated. The corrosion rate was in the order of H282 < N90 < HR160, showing an increase in the corrosion rate with corrosion time under a high-temperature and oxidative Li molten-salt environment. The rate depended on the morphology and structure of the scale in contact with the molten salt and was also particularly related to the region of pores, cracks, and Cr depletion.
Corrosion mechanism of YSZ
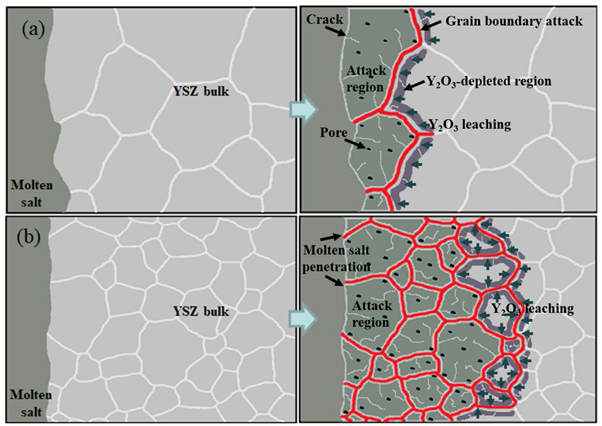
To use SOM anodes for the electrowinning of various metals, the corrosion resistance of YSZ can be improved by adjusting the grain size instead of adding stabilizers.
Reduction process
Since the Kroll process was commercialized in the 1940s, no alternative process has been reported yet. The main reason is its high affinity with oxygen and difficulty of mass production due to its high melting point above about 1700 degrees Celsius.
Therefore, research and development to overcome these problems has been actively conducted since the 1990s, and these processes can be broadly classified as thermal reduction of metal oxides using Ca metal or direct electrolytic reduction of the oxides in molten salt medium
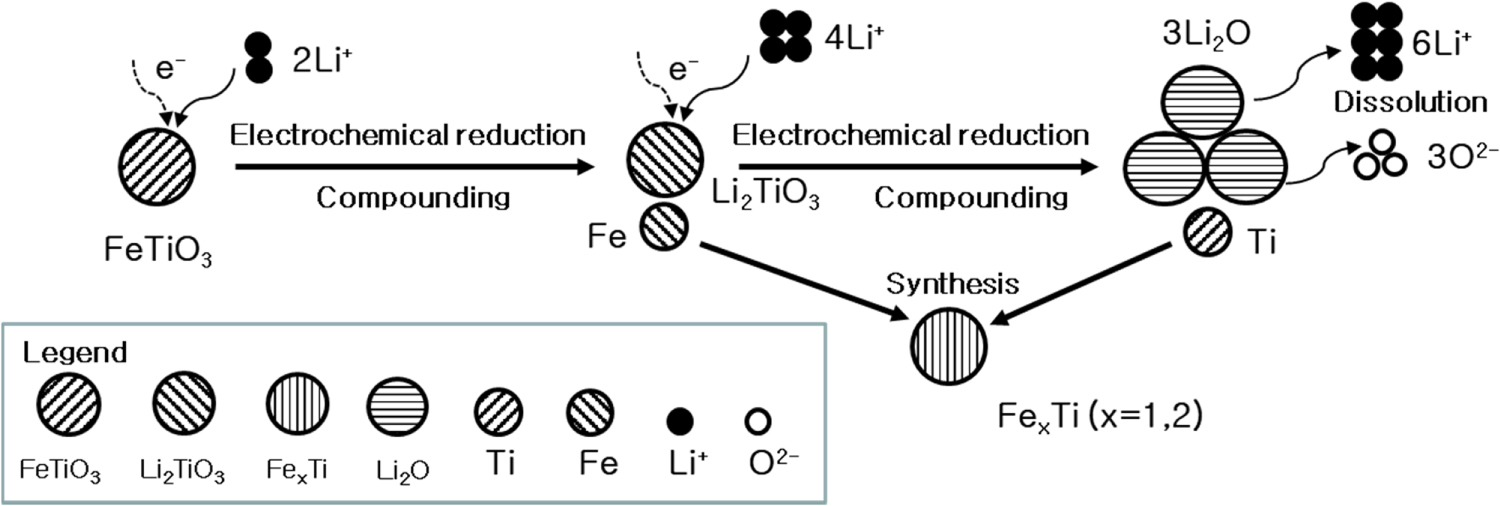
Solid state electroreduction mechanism
The electrochemical reduction mechanism of ilmenite was proposed based on our experimental results. ICP analysis detected low levels of impurities in the FeTi sample, and the overall purity of the FeTi was calculated as no less than 98.4% based on the ICP data. The mechanism of ferrotitanium flake formation was described based on the experimental findings.
Liquid state reduction mechanism
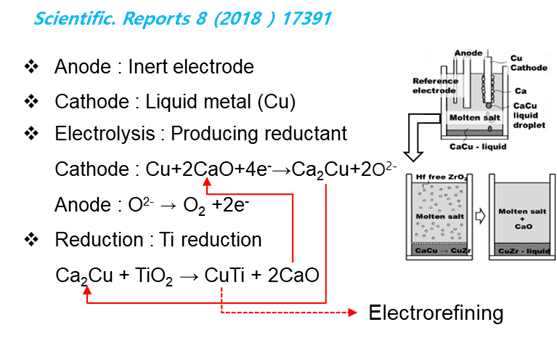
Liquid state reduction process is similar to the EMR/MSE process in which Ca reducing agent is combined with noble metal, in this case, Cu and placed in the lower part of the reactor, but the method of directly supplying the metal oxide raw material to the CaCu melt is different from other processes.
Therefore, by forming a low melting point alloy with Cu at the same time as the reduction reaction of TiO2, physical electrolyte separation is achieved by density difference after the reaction is completed. As a result, it has the advantage of being able to process in large quantities as seen in the separation process of pig iron and slag in a blast furnace.
CaO and Cu are recycled by the electrolytic process using the inert anode and the electrorefining process of CuTi respectively.
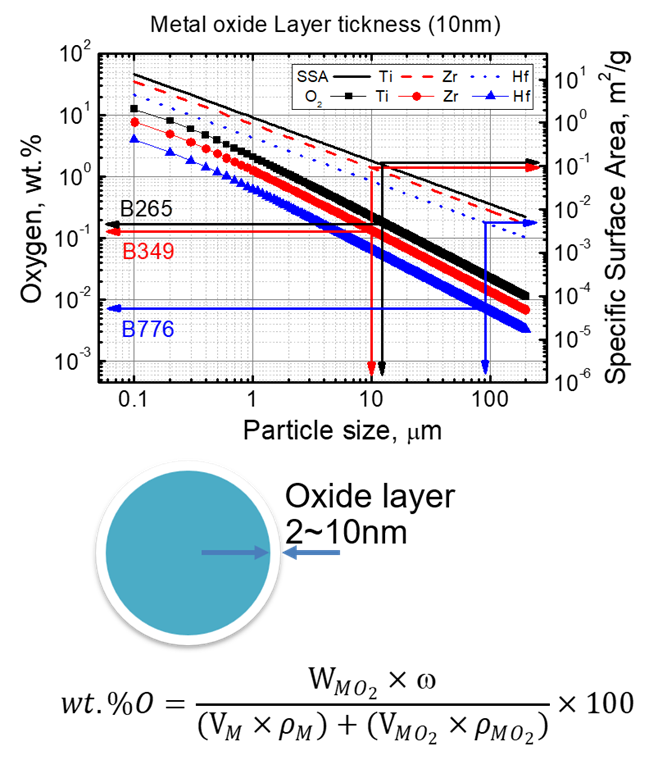
Intrinsic oxygen contamination of group IV metals
Group 4 metals are known to have an oxide film of about 2 nm to 10 nm formed on the surface. Accordingly, the smaller the particle size, the higher the ratio of oxide to the volume of the particle, and thus the amount of residual oxygen increases. Therefore, when calculated conservatively, that is, when the oxide film thickness is 10 nm, it is necessary to maintain a particle size of at least 10 micrometers for Ti and Zr and 100 micrometers for Hf in order to achieve the industrial standard residual oxygen level.